ABOUT US
Established in 2001, Equinox Biotech Co., Ltd. is a high-tech company that produces molecular biological rapid diagnostic kits, chemical drugs and membrane for biological diagnostic kits made of polymer biomedical functional materials.
We have gotten CE and ISO 13485 certificates. Our production management is strictly in accordance with the international quality management system to ensure product quality.
-
Rapid diagnostic kits
-

Membrane for biological diagnostic kits
STRATEGY AND
CULTURE
Dedicate to develop and make safe, convenient, rapid and accurate POCT products. Sincerely care for health and life.
Devote to providing professional products and services of rapid diagnosis and prenatal & postnatal care.
Work hard to realize the dream shared by employees, customers and partners with customer as the center.
PRODUCTS

Rapid Diagnostic Kits
Supply hormone tests, drug of abuse tests, infectious disease tests, tumor maker tests, etc.
Obtain ISO9001, ISO13485, CE and MDSAP certificates.

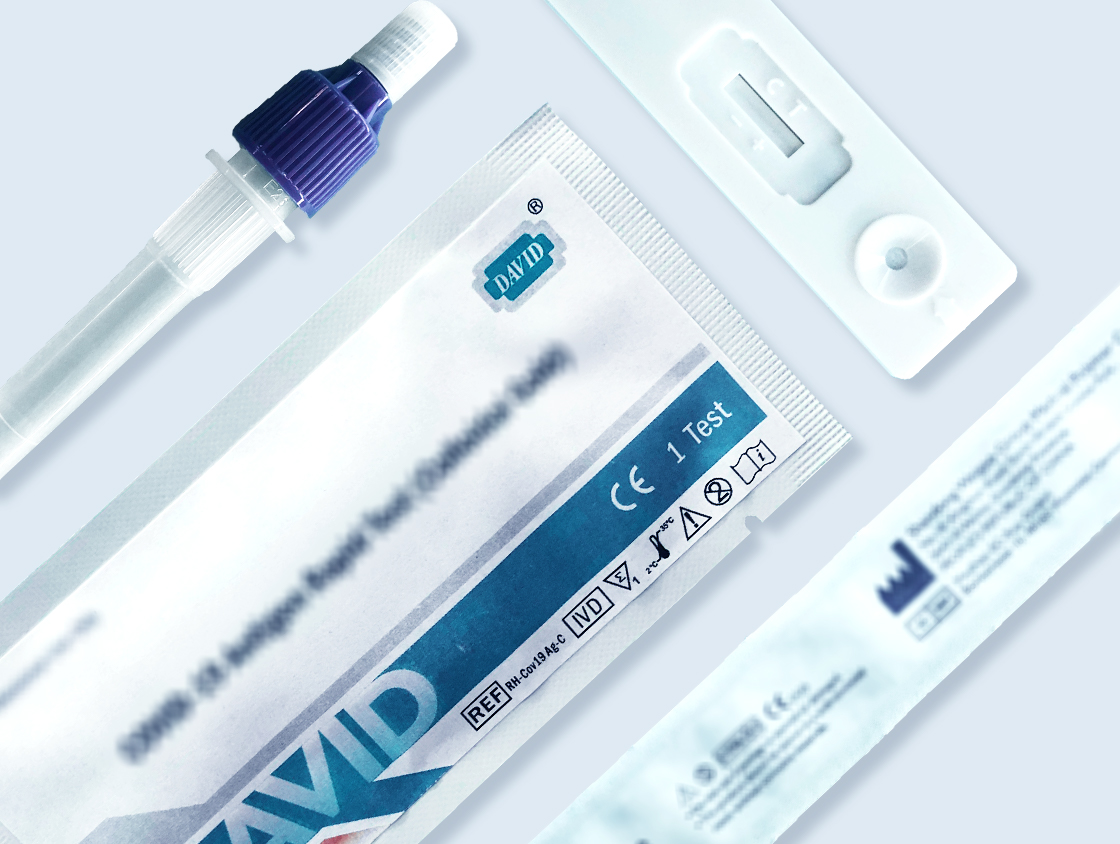
Membrane For Biological Diagnostic Kits
GLOBAL MARKET
Our products are sold domestically as well as exported to the many foreign countries. We have established a perfect industrialization system of R&D, production and marketing in the field of bio diagnosis, which provides a powerful driving force to the company's sustainable development.



